Article
Exploring New Treatment Options for Idiopathic Pulmonary Fibrosis
Author(s):
Results from a phase 2 trial, published recently in The New England Journal of Medicine, suggest that treatment with 150 mg of nintedanib slowed lung-function decline and decreased acute exacerbations in patients with idiopathic pulmonary fibrosis.
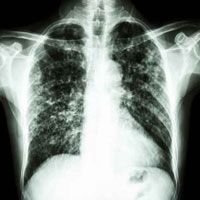
Results from a phase 2 trial, published recently in The New England Journal of Medicine, suggest that treatment with 150 mg of nintedanib slowed lung-function decline and decreased acute exacerbations in patients with idiopathic pulmonary fibrosis (IPF), a fatal lung disease typically affecting individuals older than 50 years and characterized by worsening dyspnea and progressive loss of lung function.
At CHEST 2014, Ganesh Ragu, MD; Craig Thurn, MD; and Sudhakar Pipavath, MD, discussed the clinical perspectives in IPD — primarily the safety and efficacy of nintedanib as a potential IPF treatment.
A total of 1,066 subjects were involved within 2 replicate, 52-week, phase 3 trials, IMPULSIS-1 and IMPULSIS-2. Randomly assigned, to either nintedanib or placebo, the adjusted annual rate of change in FVC was found to be -114.7 ml with nintedanib whereas a -239.9 ml with the placebo.
While the primary end point was the annual decline in forced vital capacity (FVC), the main secondary endpoints were the “time to the first acute exacerbation and the change from baseline in the total score on the St. George’s Respiratory Questionnaire," both assessed over the 52-week timeframe.
All-cause mortality did not indicate a statistically significant difference. Findings suggested the proportion of patients who died from any cause during the period of treatment was 5.5% in the nintedanib group and 7.8% in the placebo group. The most frequent adverse events were reported to be diarrhea — (61.5%) in the nintedanib group and (18.6%) in the nintedanib and placebo groups, which led to a premature discontinuation of the medication within 14 subjects (4.5%).
Within each trial, greater proportions of patients in the nintedanib group compared with the placebo group had a favorable response in FVC. The study results concluded that nintedanib was found to significantly reduce FVC decline, consistently slowing disease progression.




