Exploring treatment options for rotator cuff disorders
With recent advances in diagnostic capabilities, rehabilitation, and surgical techniques, patients with rotator cuff disease and injuries usually can expect a favorable outcome. Successful treatment requires a careful awareness of a diagnostic and treatment algorithm that takes into consideration comorbidities that may complicate a patient's rotator cuff disorder.
With recent advances in diagnostic capabilities, rehabilitation, and surgical techniques, patients with rotator cuff disease and injuries usually can expect a favorable outcome. Successful treatment requires a careful awareness of a diagnostic and treatment algorithm that takes into consideration comorbidities that may complicate a patient's rotator cuff disorder.
Several nonoperative treatment options are available that help avoid the inherent risks of surgery. However, arthroscopic evaluation and repair has become the gold standard for treatment because it allows for superior diagnostic capability and surgical versatility. Outcomes generally are favorable with a low incidence of complications, but total recovery may take longer than traditionally thought. Although most function usually is regained within 6 months, some gains in strength and motion have been documented at up to 2 years after surgery.
This 2-part article discusses and presents a rationale for patient evaluation and management of rotator cuff disease. In the first part ("Taking a closer look at rotator cuff disorders," The Journal of Musculoskeletal Medicine, October 2008, page 481), I reviewed the anatomy and pathogenesis of various types of rotator cuff disease and approaches to evaluation. This second part describes nonoperative and operative treatment options.
NONOPERATIVE TREATMENT
Weighing the risks
Although nonoperative treatment helps avoid the risks of surgery, failed nonoperative treatment results in continued or recurrent symptoms or in progression of pathology, leading to eventual surgical treatment, possibly after irreversible changes have occurred in the rotator cuff. When counseling patients about treatment options, it is important to characterize a cuff disorder with respect to the patient's age, the tear size, the injury mechanism, chronicity, and muscle atrophy/fatty infiltration. Within this framework, the overall risk of irreversible changes to the cuff with continued nonoperative treatment is weighed against the potential for improvement with continued nonoperative treatment.
Tendinitis or partial-thickness tears probably will improve rapidly with nonoperative treatment. Therefore, a period of nonoperative treatment may be offered to a patient with little risk of progression of the pathology. In addition, a 67% success rate with nonoperative management of these conditions has been reported, with only 18% recurrence for an average of 2 years.1
Small to medium-size tears (2 to 3 cm), existing tears with a recent loss of function, and full-thickness tears of any size in a young, active person (younger than 60 years) are at risk for progression if early nonoperative therapy is not successful. In this age-group, prolonged nonoperative treatment has a low success rate and carries a risk of leading to irreversible changes in the tendon and muscle or progression of the tear, which may complicate the eventual surgical repair. For these patients, early surgical treatment after a short course of non-operative treatment is preferable.
Patients older than 70 years with large chronic tears probably already have experienced irreversible changes in their cuff, leading to loss of function or pain or both. For these patients, there is less risk associated with attempting prolonged nonoperative treatment with the goal of controlling a patient's pain and improving function.
An estimated 70% to 80% of rotator cuff tendinitis cases may be managed successfully with a nonoperative approach if the condition is managed early in its course. In a series of 616 patients, 78% treated within 4 weeks of symptoms onset had a successful outcome, versus 67% of those who had symptoms for 6 months or longer.1 Acromial morphology played a role in this study; patients with a type 1 (flat) acromion had the highest rate (91%) of successful nonoperative treatment, compared with 68% and 64% success rates for patients with type 2 and type 3 acromions, respectively.
Nonoperative methods for managing rotator cuff tendinitis and impingement syndrome include corticosteroid injections, oral anti-inflammatory medication, activity modification, ultrasonography and phonophoresis, and rehabilitation that emphasizes stretching and strengthening of the rotator cuff and scapular musculature. Although these therapeutic options are the mainstay of nonoperative treatment, there are few controlled studies that provide objective data on them.
Corticosteroids
These agents have been shown to be effective in controlling symptoms associated with rotator cuff pathology, often obviating the need for surgical treatment.2 Corticosteroid injections control pain and improve function better than lidocaine injections alone. However, these injections carry an inherent risk of causing necrosis and fragmentation of the tendon tissue, making surgical repair more difficult.2 Therefore, corticosteroid injections into the subacromial space to manage rotator cuff tendinitis or symptomatic small tears should be used with discretion. We recommend a guideline of limiting a series of injections to no more than 3 injections given at 3-month intervals.
Physical therapy rehabilitation
This is an important part of nonoperative treatment. After a brief (eg, 3-day) rest period to decrease acute inflammation and pain, the patient should be asked to engage in a stretching protocol to increase range of motion, especially patients who have lost internal rotation, because the tight posterior capsule can elevate the humeral head and cuff into the acromion. After improvement in range of motion is achieved, the patient should engage in regular light exercise to strengthen the muscles of the rotator cuff and scapular stabilizers in addition to deltoid and trapezius strengthening. Strengthening the rotator cuff and scapular stabilizers improves shoulder kinematics, and a strong deltoid improves abduction strength, as long as the rotator cuff provides a stable fulcrum.
For patients to benefit from rehabilitation, compliance with a regular program is essential. Because supervised physical therapy improves compliance, there is a benefit to having supervision. However, in a study of patients who had undergone arthroscopic subacromial decompression without rotator cuff repair, there was no difference in outcomes between the group in supervised therapy and the group in a self-directed program.3 Enrollment in the study may have heightened patients' awareness of the protocols and increased the compliance of the self-directed group.
Supervised physical therapy often uses other modalities, such as ultrasonography, phonophoresis, and iontophoresis, to manage subacromial bursitis, cuff tendinitis, and small symptomatic cuff tears. Ultrasonography is a common physical therapy modality that often is applied to the nonoperative management of rotator cuff tendinitis and subacromial impingement. High-frequency ultrasonography (10 to 30 MHz) creates a thermal effect and, possibly, a mechanical effect that can increase blood flow to a focal area, theoretically augmenting a tissue's capacity to heal. Although no studies demonstrate effectiveness in the management of rotator cuff tendinitis or subacromial impingement symptoms, ultrasonography is used frequently.
Twenty patients with subacromial bursitis in a prospective, double-blind placebo-controlled study were randomized to receive sham or real ultrasonography 3 times a week for 4 weeks.4 There was no demonstrable benefit in the ultrasonography group in terms of pain ratings, function, or time to recovery. It may be that the therapeutic benefit was small and was not detected with low numbers of participants over a short period.
Phonophoresis uses ultrasonography to deliver medication directly into superficial tissues. It is postulated that the mechanical and thermal effects of ultrasonography together increase tissue permeability and even cellular permeability.
Iontophoresis is another modality in which physical properties of electric current are used to deliver medication, usually corticosteroids, directly to pathological tissues. Much recent work has been focused on determining the optimal physical parameters for maximal medication delivery; validated outcome studies are not yet available.
SURGICAL TREATMENT
Even if they have received appropriate nonoperative care, patients with rotator cuff pathology often require surgical treatment. Providing appropriate treatment requires a thorough understanding of the specific pathological condition to be managed. Even with adequate imaging studies, conducting an arthroscopic diagnostic evaluation of the glenohumeral joint and the subacromial space is the best way to define the pathoanatomy and identify other problems. Intraoperative decisions often need to be made as anatomical details of the pathology are revealed.
Arthroscopic evaluation and management of subacromial bursitis and rotator cuff tears may be performed in either the beach chair or the lateral decubitus position. Use of the beach chair position prevents potential distortion of normal anatomy and allows the surgeon to frequently reposition and examine the arm during the case. The lateral position distracts the arm, which can enlarge the subacromial space and the glenohumeral space. Because the lateral position holds the arm in a fixed abducted position, the arm should be removed from traction to allow for inspection in several positions. Care must be taken to avoid repair of a cuff tear in excessive abduction, because this repair will be under additional tension when the arm is in a neutral position.
Diagnostic arthroscopy
Surgical management of rotator cuff injuries starts with a thorough diagnostic arthroscopy of the patient's entire glenohumeral joint and subacromial space. With visualization of the glenohumeral joint from the posterior portal, a standard anterosuperior portal is placed within the rotator interval to allow for outflow and instrumentation.
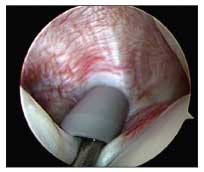
Figure 1 – This arthroscopic view shows an inflamed long head of the biceps tendon, a common source of anterior shoulder pain.
The subscapularis insertion is evaluated in various degrees of arm rotation; particular attention is paid to its confluence with the glenohumeral ligament complex comprising the biceps sling. The biceps tendon is pulled into the joint and inspected for erythema or fraying, which is indicative of biceps tendinitis (Figure 1), or a torn biceps tendon (Figure 2), which may suggest the need for biceps tenodesis or tenotomy. The articular side of the supraspinatus is carefully inspected with the arm abducted, externally rotated, and forward elevated. The rotator cuff cable is identified, and an absorbable monofilament suture is passed through a spinal needle to mark focal irregularities or small tears in the articular side of the cuff tendon, which will facilitate bursal-side inspection.
Subacromial decompression
The subacromial space is entered posteriorly, and a thorough bursectomy is performed. Visualization is maximized by maintaining hemostasis through careful dissection using radiofrequency coagulation and careful fluid management in the setting of effective intraoperative blood pressure control. Besides providing visualization, bursal debridement alone can relieve pain; pain fibers are known to exist in this structure.5,6
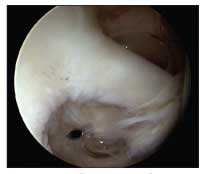
Figure 2 – An arthroscopic view shows a torn biceps sling causing medial subluxation of the long head of the biceps tendon. This common source of pain often requires tenotomy or tenodesis.
If an acromioplasty is to be performed, the coracoacromial ligament insertion on the anterolateral corner of the acromion is identified and elevated with a radiofrequency device introduced from the lateral portal. The decision to perform a routine acromioplasty is a source of controversy. Often an acromioplasty is needed to maximize visualization, ensure an absence of cuff impingement, and reduce symptoms.
Arthroscopic rotator cuff repair
Arthroscopic preparation and repair of injured rotator cuff tendons allows for accurate identification of the tear pattern and recognizing subtleties of delamination and tissue adhesions that are not detected with an open repair. To accurately repair a torn rotator cuff, it is crucial to understand the anatomy of the tear and ensure the mobility of the tendon, remaining aware that large tears are pulled posteriorly.
Repairing partial-thickness tears
When conservative management of a partial-thickness tendon tear has not been successful, arthroscopic evaluation provides crucial evaluation of the thickness of the articular- and bursal-sided tears. This is especially true with respect to articular-sided tears, which cannot be fully appreciated without an arthroscopic evaluation of the glenohumeral joint.
Early studies of arthroscopic debridement and acromioplasty alone as treatment of low-grade (less than 25% thickness) partial-thickness tears reported 76% to 89% good to excellent results.7-9 However, several authors recommend surgical repair of partial-thickness tears,10-12 especially if they make up 50% or more of the tendon thickness; there is a lower threshold to repair bursal-sided tears.13
Open/"mini-open" rotator cuff repair
Before the advent of arthroscopic shoulder surgery, rotator cuff tears were identified and repaired with an open exposure of the subacromial space, requiring detachment of the anterior deltoid from the acromion, which required meticulous repair. Dehiscence of the deltoid from the acromion causes significant debilitation, and the value of minimizing deltoid intra-operative injury has since been recognized. In an effort to limit damage to the deltoid, mini-open techniques have been developed. These techniques allow for open exposure to the subacromial space by splitting the deltoid without detachment from the acromion.
Postoperative care, recovery, complications
Postoperative rehabilitation is conducted in 3 phases. The first phase includes a protected 6-week period of tendon healing. During this time, the patient wears a sling full-time, removing it only for hygiene and supervised physical therapy. During this phase, range of motion goals are 140° of forward flexion, 40° of external rotation with the arm at the side, and 60° of abduction in neutral rotation. I do not recommend early use of canes or pulleys, because these are active assist devices.
Active motion begins during the second phase, from week 6 to week 12, with a gradual increase in the range of motion. During this intermediate phase, gentle stretching is permitted at the end of ranges of motion. Although active motion is allowed, no strengthening or resisted motion is allowed. However, isometrics with the arm at the side in a neutral position is allowed at 8 weeks.
The third phase begins at 3 months after surgery and continues until maximum improvement is achieved. Full range of motion is a goal during this period. Strengthening exercises progress from isometrics to light resistive bands to weights ranging from 1 to 5 lb. It is important to strengthen scapular stabilizers and the deltoid in addition to rotator cuff muscles. I recommend that strengthening work be performed only 3 times per week to avoid cuff tendinitis.
The strengthening program evolves to include plyometrics and sport-specific simulation training as the patient is able to tolerate. At 6 months, light throwing is allowed. Because a durable repair requires a tendon-to-bone healing that can withstand dynamic loading, participation in collision sports and hard pitching from a mound usually are not permitted until 9 months. Full recovery and maximum performance gains are expected at 1 year, although there is evidence of statistically significant functional gains during the second postoperative year.14
Results and outcomes
The success of surgical management of rotator cuff pathology depends on the anatomy and classification of the tear, the biology and physiology of the tissue, the quality of the repair, and associated comorbidities, as well as several medical and sociological factors.15 The results of arthroscopic repair of full-thickness tears are at least equal, if not superior, to those of open repair. Studies have shown patient satisfaction as high as 98%, with low rates of patient morbidity and pain.16-18
Massive retracted U-shaped tears can be mobilized and repaired via "margin convergence," using side-to-side stitches to convert large U-shaped tears to smaller C-shaped tears, which are then repaired to bone in a standard fashion.19 Patient satisfaction as high as 100% with 96% good to excellent results for arthroscopic repair of medium-sized tears (3 cm) has been reported.16,20
Complications
The major postoperative complication is failure of the tendon to heal, whether it was managed arthroscopically or using the open technique. Many biological, anatomical, and behavioral factors relate to the probability that a tear will heal after repair. To increase the probability of healing, I recommend frequent use of side-to-side margin convergence to improve the biomechanics and multiple anchors to augment the tendon-to-bone repair.
Deltoid dehiscence is a rare but devastating complication of formal open management of rotator cuff tears. This generally can be avoided by not removing the lateral acromion and by performing a secure, anatomical deltoid repair.21
Infections occur in fewer than 1% of patients. Infections may result from Staphylococcus aureus or a more virulent organism, such as Proprionibacterium, coagulase-negative Staphylococcus, or Peptostreptococcus. Management of infections includes early detection, debridement, and proper use of antibiotics. In properly managed infected rotator cuff repairs, a good to excellent result may be expected in about one-third of patients.
Shoulder stiffness is a potential complication in an arthroscopic repair that can be minimized by avoiding the pain and morbidity associated with an incision and deltoid violation. Early postoperative loss of range of motion usually can be overcome with appropriate rehabilitation.
References:
References
1.
Morrison DS, Frogameni AD, Woodworth P. Non-operative treatment of subacromial impingement syndrome.
J Bone Joint Surg
. 1997;79A:732-737.
2.
Blair B, Rokito AS, Cuomo F, et al. Efficacy of injections of corticosteroids for subacromial impingement syndrome.
J Bone Joint Surg
. 1996;78A:1685-1689.
3.
Andersen NH, Søjbjerg JO, Johannsen HV, Sneppen O. Self-training versus physiotherapist-supervised rehabilitation of the shoulder in patients treated with arthroscopic subacromial decompression: a clinical randomized study.
J Shoulder Elbow Surg
. 1999;8:99-101.
4.
Downing DS, Weinstein A. Ultrasound therapy of subacromial bursitis: a double blind trial.
Phys Ther
. 1986;66:194-199.
5.
Gotoh M, Hamada K, Yamakawa H, et al. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases.
J Orthop Res
. 1998;16:618-621.
6.
Soifer TB, Levy HJ, Soifer FM, et al. Neurohistology of the subacromial space.
Arthroscopy
. 1996;12:182-186.
7.
Esch JC, Ozerkis LR, Helgager JA, et al. Arthroscopic subacromial decompression: results according to the degree of rotator cuff tear.
Arthroscopy
. 1988;4:241-249.
8.
Snyder SJ, Pachelli AF, Del Pizzo W, et al. Partial thickness rotator cuff tears: results of arthroscopic treatment.
Arthroscopy.
1991;7:1-7.
9.
Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears.
Orthopedics
. 2003;26:387-390.
10.
Fukuda H. The management of partial-thickness tears of the rotator cuff.
J Bone Joint Surg
. 2003;85B:3-11.
11.
Lehman RC, Perry CR. Arthroscopic surgery for partial rotator cuff tears.
Arthroscopy
. 2003;19:E81-E84.
12.
Lo IK, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff.
Arthroscopy
. 2004;20:214-220.
13.
Fukuda H. Partial-thickness rotator cuff tears: a modern view on Codman's classic.
J Shoulder Elbow Surg
. 2000;9:163-168.
14.
Cole BJ, McCarty LP 3rd, Kang RW, et al. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up.
J Shoulder Elbow Surg
. 2007;16:579-585.
15.
Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears: gender, age, and other factors affecting outcome.
Clin Orthop Relat Res
. 1999;(367):243-255.
16.
Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up.
J Shoulder Elbow Surg
. 2002;11:19-24.
17.
Tauro JC. Arthroscopic rotator cuff repair: analysis of technique and results at 2- and 3-year follow-up.
Arthroscopy
. 1998;14:45-51.
18.
Gartsman GM, Khan M, Hammerman SM. Arthroscopic repair of full-thickness tears of the rotator cuff.
J Bone Joint Surg
. 1998;80A:832-840.
19.
Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair.
Arthroscopy
. 2001;17:905-912.
20
. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up.
Arthroscopy
. 2003;19:131-143.
21.
Sher JS, Iannotti JP, Warner JJ, et al. Surgical treatment of postoperative deltoid origin disruption.
Clin Orthop Relat Res
. 1997;(343):93-98.