Improving Vitiligo Repigmentation With Emerging Targeted Therapies
Vitiligo is a chronic pigmentary disorder characterized by white lesions on the skin that result from the loss of functional melanocytes in the skin and/or hair.1 It is the most prevalent skin depigmentation disorder worldwide, occurring in approximately 1% of the global population and 1 million to 2 million individuals in the United States.1,2 Although it is often more apparent in people with dark skin, its prevalence is similar across sexes, ethnicities, and geographic regions.3 Vitiligo presents mostly in patients who are younger than 30 years, and spontaneous regression is rare.3
Vitiligo presents as either nonsegmental vitiligo (often-symmetrical white patches) or segmental vitiligo (white patches that distribute unilaterally and typically follow a dermatome).4 Rarely, both types can exist in a patient simultaneously. Nonsegmental vitiligo is the most prevalent, affecting up to 90% of vitiligo patients. It usually develops gradually in adulthood, whereas segmental vitiligo often occurs earlier and with a more-rapid onset.4 Segmental vitiligo is more common among children with vitiligo, affecting approximately 30% of this population.4 This type of vitiligo can respond poorly to standard treatment and may require surgery to achieve
optimal repigmentation.4
Severity of vitiligo is commonly assessed using the vitiligo area severity index (VASI).5 The VASI calculates disease involvement using hand units, with 1 hand unit corresponding to approximately 1% of the total body surface area (BSA). The degree of pigmentation is rounded to the following percentages: 100% (full depigmentation), 90% (VASI90; small areas of pigmentation), 75% (VASI75; depigmented areas larger than the pigmented area), 50% (VASI50; similar areas of pigmentation and depigmentation), 25% (VASI25; pigmented area larger than the depigmented area), and 10% (VASI10; small areas of depigmentation). The vitiligo disease activity score, another scale used to assess vitiligo, is based on patient perception of disease activity.5
For vitiligo treatment, several off-label options have offered varying degrees of therapeutic success among patients.3,6 Conventional strategies have involved combinations of phototherapy with topical formulations of corticosteroids, calcineurin inhibitors, or vitamin-D analogues, although these approaches typically have limited success in treating segmented vitiligo.3,4,7 In July 2022, the topical JAK 1/2 inhibitor ruxolitinib became the first FDA-approved treatment for vitiligo, based on pivotal phase 3 efficacy and safety data.8 Other emerging treatment options, such as tofacitinib, have shown promise in results from clinical studies.9,10
This article reviews the negative effects of vitiligo often experienced by patients, recent advances in the knowledge of vitiligo pathogenesis, and the current and emerging treatment modalities that may be used to reverse depigmentation and restore melanin.
CLINICAL AND PSYCHOSOCIAL IMPACT
Dermatologists often consider vitiligo to be a relatively benign condition that does not have a meaningful response to treatment.11 However, itching and/or burning has been reported in approximately 35.1% of patients with vitiligo, typically those with lesions affecting greater than 25% of the BSA.12
The psychosocial effects of this skin condition can be substantial, with lesions causing shame, anxiety, depression, and impaired quality of life (QOL).3,12 Results from a prospective questionnaire-based study assessing the extent of vitiligo and accompanying impairments in QOL showed that the dermatology life quality index (DLQI) score, which assesses health-related QOL in patients with skin disease, showed greater QOL impairment in patients with an affected BSA greater than 25% (adjusted odds ratio [aOR], 2.17; 95% CI, 1.71-2.75; P <.001) and in those with a greater number of body parts affected (tertile 2: aOR, 1.47 [95% CI, 1.10-1.96; P < .001]; tertile 3: aOR, 2.18 [95% CI, 1.64-2.89; P < .001]).12,13 Patients with an affected BSA greater than 25% were more self-conscious about their lesions and less capable of participating in activities of daily living (eg, shopping; both P < .001); they also altered their clothing choices and had more impairments of socializing or leisure activities, participation in sports activities, and relationships and sexual function (all P < .001).12
Several autoimmune conditions often accompany vitiligo, and they may contribute to substantial morbidity in affected patients.6 Systemic autoimmune conditions are typically associated with nonsegmental vitiligo, although local autoimmune skin conditions, such as linear morphea, are often seen with segmental vitiligo.4,14 Results from a retrospective analysis of hospital medical records dating from January 1, 2000, to June 21, 2011, within the Research Patient Data Repository showed that 23% of patients with vitiligo also had autoimmune conditions.6 Thyroid disorders were most common (11.8%), followed by psoriasis (7.6%), rheumatoid arthritis (2.9%), alopecia areata (2.4%), inflammatory bowel disease (2.3%), systemic lupus erythematosus (2.2%), and type 1 diabetes (0.8%). The authors noted that the incidence of autoimmune conditions among patients with vitiligo may be higher than shown in the study’s results, as many of these conditions can be subclinical.6
Vitiligo has substantial clinical and psychosocial effects; therefore, knowledge of its underlying mechanisms is important to develop therapeutic and patient care approaches.3
PATHOGENESIS AND REPIGMENTATION
Melanogenesis
Melanin, a natural pigment derived from dopaquinone that protects the skin from UV radiation (among other functions), is produced in the melanosomes in melanocytes.1 Several paracrine cytokines (such as α-melanocyte–stimulating hormone, stem cell factor, endothelin-1, nitric oxide, corticotropin, prostaglandins, thymidine dinucleotide, and histamine) contribute to melanogenesis by activating pigment-related proteins, including MITF, TYR, TRP1, and TRP2.1 MITF is a key regulator of melanogenesis across every pathway; it upregulates TYR, TRP1, and TRP2 and manages melanocyte differentiation, pigmentation, proliferation, and cell survival.1 The primary role of TYR, TRP1, and TRP2 is to convert tyrosine into melanin pigment; however, TYR is the only enzyme specifically needed for melanogenesis.1
Pathogenesis
The causes and pathogenesis of vitiligo are not fully understood, but many theories have been suggested; some involve the immune system, genetics, stress, the nervous system, or biochemical drivers.1 The loss of melanocytes and resultant depigmentation may occur through interference in pathways involved in melanin production and an autoimmune-mediated breakdown of melanocytes, during which immune cells attack melanocytes.1,3,15 Notably, the melanocyte immune response is more targeted in segmental vitiligo than it is in nonsegmental vitiligo, and the inflammatory response is brief and locally confined. The differences in pathogenesis between the 2 types of vitiligo account for the higher rate of systemic autoimmune disorders associated with nonsegmental vitiligo.14
Similar to other autoimmune inflammatory diseases, such as rheumatoid arthritis and alopecia areata, the pathogenesis and cytotoxicity in vitiligo are driven by activated CD8-positive (CD8+) T cells.15,16 These cells are recruited to melanocytes through a signaling pathway regulated by JAK1 and JAK2, in which interferon-γ (IFN-γ) induces chemokines CXCL9 and CXCL10.16 Both CXCL9 and CXCL10 are considered to be biomarkers of disease activity, as their serum levels are significantly elevated in patients with vitiligo (P < .01) and are higher among patients with progressive disease than they are in those with stable disease.17 The serum CXCL10 level also increases with higher VASI scores among patients with progressive disease and decreases among patients who respond to treatment.17 The IFN-γ–chemokine pathway is involved in the initiation, progression, and maintenance of vitiligo lesions; therefore, therapeutic targeting of the IFN-γ–chemokine axis may be a promising method of treatment for both segmental and nonsegmental vitiligo.3,16
Repigmentation
In contrast to many other autoimmune diseases, vitiligo can be fully reversed. Melanocytes within immune-privileged sites of the body (eg, hair follicles, brain, eye, and inner ear) are often unaffected, and hair follicles within the epidermis have melanocyte stem cells that can repopulate lesion areas with newly differentiated melanocytes that can repigment the skin.3 Clinical repigmentation most often occurs in a punctate, perifollicular pattern characterized by small, round spots of pigmentation that surround hair follicles.7 Repigmentation can also occur in a marginal pattern, in which functional melanocytes at the borders of epidermal lesions become activated and form a rim of repigmentation (ie, in a diffuse pattern) that is characterized by general darkening across the lesion areas. A combined pattern describes an area where repigmentation does not occur in a single prespecified pattern or occurs in more than 1 pattern (FIGURE).7
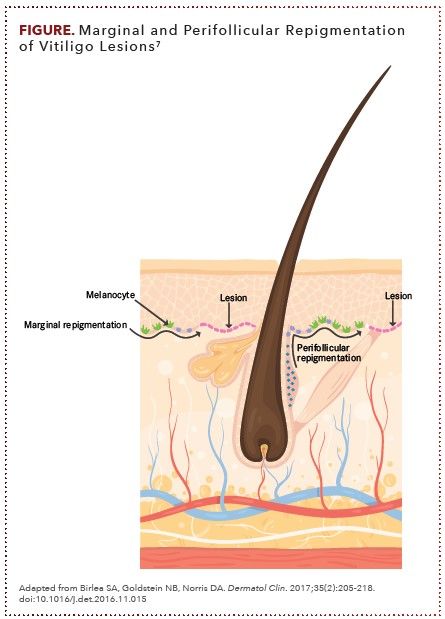
Affected areas with higher numbers of hair follicles (eg, face, arms, forearms, thighs, legs, abdomen,and back) tend to have a more rapid and effective repigmentation response to treatment. By contrast, response rates are low (and tend to be slow and incomplete if response occurs) in depigmented areas that have a low density or lack of hair follicles (eg, hands, fingers, feet, toes, volar wrists, genital sites, and mucosal or semimucosal surfaces).7
These areas achieve a treatment response from the pool of melanocytes at the borders of the lesion, epidermal melanocytes that may be located in the middle of depigmented lesions, or melanocytes that originate from the extrafollicular dermis. Areas of depigmentation with leukotrichia (white terminal hairs), which may indicate an ongoing attack on melanocytes by active CD8+ T cells, tend to respond poorly to treatment and are unlikely to undergo repigmentation.7
Assessing Treatment Response
Current methods used to assess response to treatment are highly subjective.18 The physician’s global assessment (PGA) is commonly used. However, PGA scores have high interrater and intrarater variation, because they primarily depend on the visual judgment of the rater (ie, the provider). Furthermore, detection of repigmentation within lesions using the PGA score can take several months, which makes the PGA score an inadequate tool for assessing treatment effectiveness over a short time period.18
OVERVIEW OF TREATMENTS FOR VITILIGO
Successful therapeutic repigmentation in vitiligo involves both immunosuppression to halt depigmentation and restoration of stem cell melanocytes in the hair follicle to promote repigmentation.3,7 Most treatments currently used for vitiligo target both of these objectives; however, the relative extent of stimulation needed to suppress the autoimmune response and regenerate melanocytes to achieve maximal reversal of vitiligo is unknown.7
Conventional Therapeutic Strategies
PHOTOTHERAPY
UV radiation light—delivered primarily as narrowband UVB (NBUVB) light, broadband UVB (BBUVB) light, or psoralen with UVA light (PUVA)—provides the strongest stimulation to melanocyte precursors and the highest rate of repigmentation of all therapies.7 In a meta-analysis, more patients who received NBUVB phototherapy achieved greater than 75% repigmentation (63%; 95% CI, 50%-76%) than did those who received BBUVB phototherapy (57%; 95% CI, 29%-82%).19 PUVA and NBUVB light both induce immunosuppression and promote melanocyte proliferation and pigmentation; however, use of NBUVB light has resulted in better repigmentation of lesions with fewer toxicities.3
Results from a randomized, open, prospective study of 50 patients showed that NBUVB light yielded a higher mean degree of repigmentation than did trimethylpsoralen PUVA when the results of therapy-resistant sites were excluded (67.57% over a mean treatment duration of 6.3 months vs 54.2% over a mean treatment duration of 5.6 months, respectively; P = .007), as well as a higher percentage of patients who achieved more than 75% repigmentation (54.16% vs 20.83%; P = .018).20
Results from another double-blind, randomized trial (N = 50) showed that after at least 48 phototherapy sessions, percentage improvement in BSA was achieved by more patients with vitiligo who were treated with NBUVB light than by those treated with PUVA (21 vs 13 patients; z score, 2.7; P = .007), and the skin color match between affected and unaffected skin was excellent for all patients given NBUVB light but for only 44% of patients given PUVA (χ2 = 19; P < .001).21 Additionally, more patients who received PUVA therapy (96%) developed erythema during treatment compared with those who received NBUVA light (68%; χ2 = 6.6; P = .02). In addition, PUVA therapy also had logistical disadvantages—including the need for eye protection during and after treatment; contraindications in patients who are pregnant, have hepatic impairment, or are taking warfarin or phenytoin; and the need to administer psoralen before phototherapy—which can be burdensome. A possible link between PUVA and development of nonmelanoma or melanoma skin cancer was suggested, although the incidence appears to be rare among patients with vitiligo.21 Therefore, NBUVB light is generally a preferred modality of phototherapy for vitiligo.3
CORTICOSTEROIDS
The suggestion that steroid therapies stimulate differentiation of melanocytes was based on clinical observations showing temporary melasma of the face and increased pigmentation of areolae, linea alba, and perineal skin during pregnancy (which is associated with high levels of sex steroid hormones); hyperpigmentation of the face with use of estrogen-containing oral contraceptives; and increased pigmentation of the genitals, areolae, and linea alba with use of estrogen-containing ointments in infants.7 Early data showed that systemic steroid therapy led to clinical improvement in 29 of 37 patients and a significant decrease in antibody-mediated melanocyte cytotoxicity (P = .0243), suggesting that steroids prevent damage to melanocytes around the vitiligo lesions and in the hair follicles within the lesions.22 Results from a 1998 meta-analysis of nonsurgical repigmentation therapies in patients with vitiligo showed that topical class-3 corticosteroids (ie, classified as “potent” [betamethasone valerate, halometasone]) and topical class-4 corticosteroids (ie, classified as “very potent” [clobetasol propionate]) yielded the highest percentage of patients with “localized” vitiligo (affecting < 20% of total BSA) who achieved more than 75% repigmentation (class-3 corticosteroids, 56% [95% CI, 50%-62%]; class-4 corticosteroids, 55% [95% CI, 49%-61%]).19 Pooled ORs obtained using a random-effects model found that class-3 corticosteroids were the only therapy that had a significant effect over placebo for localized vitiligo (pooled OR, 14.32; 95% CI, 2.45-83.72).19
Outcomes from a 2015 Cochrane systematic review of vitiligo therapies showed that percentage repigmentation (> 75%) was higher with topical hydrocortisone plus excimer laser therapy than with excimer laser use alone (risk ratio, 2.57; 95% CI, 1.2-5.5), with application of clobetasol propionate than with methoxsalen plus sunlight (relative risk, 4.7; 95% CI, 1.14-19.39), and with betamethasone oral minipulse (OMP) therapy plus NBUVB than with OMP therapy alone (risk ratio, 7.41; 95% CI, 1.03-53.26).23
CALCINEURIN INHIBITORS
Topical calcineurin inhibitors (eg, tacrolimus, pimecrolimus) exert therapeutic effects by restoring the cytokine network that is altered in vitiligo.7 Tacrolimus downregulates transcription of genes that encode for proinflammatory cytokines (ie, IL-2, IL-3, IL-4, IL-5, IFN-γ, TNF-α, and GM-CSF), thereby hindering T-cell activation. Tacrolimus may also have a role in migration and differentiation of melanocytes during repigmentation.7
Results from a meta-analysis of prospective controlled trials showed that calcineurin inhibitors were more therapeutically effective for vitiligo than placebo or no treatment as measured by “lesion report” (ie, number of vitiligo lesions in patients having > 75% repigmentation; relative risk, 21.0 [95% CI, 1.31-336.75; P = .03] vs 2.62 [95% CI, 1.39-4.93; P = 0.003], respectively) and “patient report” (ie, number of patients having > 75% repigmentation; relative risk, 6.48 [95% CI, 0.98-42.99; P = .05] vs 1.42 [95% CI, 0.87-2.31; P = .14]).24 Calcineurin inhibitors were most therapeutically effective when used with phototherapy as measured by lesion report (relative risk, 2.08; 95% CI, 1.35-3.20; P = .001) and patient report (relative risk, 1.29; 95% CI, 0.88-1.89; P = .20). The authors attributed the difference between lesion and patient report in the subgroup given calcineurin inhibitors plus phototherapy to the lower number of lesions on the foot and hand (areas that often respond poorly to treatment) in the lesion report (P = .0056).24
Findings from a review of literature data showed that a greater proportion of patients achieved at least 75% repigmentation with tacrolimus 0.1% ointment plus excimer laser therapy than with placebo plus excimer laser therapy, including in areas generally believed to be resistant to UV phototherapy (eg, bony prominences and extremities).25 However, the outcomes of adding tacrolimus 0.1% ointment to NBUVB phototherapy were inconsistent among the studies included in the review, with the results of 1 double-blind study showing a greater reduction in the mean size of lesions with use of the combination and another double-blind study showing no difference in efficacy compared with placebo plus NBUVB phototherapy.25
VITAMIN-D ANALOGUES
Treatment with topical vitamin-D analogues (eg, calcipotriol and tacalcitol) also may improve the repigmentation response by increasing immunosuppression in the skin, which inhibits the autoimmune process locally, and by stimulating melanocyte precursors and pathways involved in melanogenesis.7 When used to treat vitiligo, vitamin-D analogues are typically combined with topical corticosteroids or phototherapy. Results from a Cochrane systematic review showed that calcipotriol plus PUVA yielded a higher rate of repigmentation compared with PUVA alone (paired OR, 4.25; 95% CI, 1.43-12.64).7,23 However, vitamin-D analogues have not been effective when given alone to manage vitiligo.7
SURGICAL PROCEDURES
Segmental vitiligo generally responds poorly to NBUVB.4 If other therapeutics also fail, surgical approaches such as minigrafting and autologous epidermal cell transplantation may be considered. Melanocytes that have been transplanted have a higher likelihood of survival in patients with segmental vitiligo than in those with nonsegmental vitiligo due to the ability to harvest from larger areas skin that are unaffected.4 Results from a retrospective review showed that autologous melanocyte-keratinocyte transplantation procedures resulted in long-term repigmentation of vitiligo lesions, with “repigmentation” defined as pigment maintained or increased for 12 months following the procedure. There was a > 75% repigmentation in 71% of anatomically based lesions (ABLs) in segmental vitiligo and in 54% of ABLs in nonsegmental vitiligo, which was maintained between 12 through 72 weeks (n = 63; ABLs = 157). The repigmentation outcomes were consistent across skin phototypes and anatomic region, including areas that are typically difficult to treat.26
JAK Inhibitors as FDA-Approved and Emerging Treatment Options
Conventional treatment options have notable limitations, including their lack of effectiveness on all areas of the body and their inconvenience (eg, need for repeat visits to a dermatologist with phototherapy and 2 applications/day with topical therapies).3 Additionally, the durability of response to conventional treatment options is often poor, with a relapse rate of up to 40% during the first year following treatment discontinuation.3 Therefore, more effective treatments for vitiligo are needed. The JAK 1/2 inhibitor ruxolitinib offers a newly approved targeted treatment option for nonsegmental vitiligo, and additional targeted therapies with promising efficacy and tolerability are currently being developed based on an improved understanding of the pathogenesis of vitiligo.3,8
PROPOSED MECHANISMS
The JAK family has 4 members (JAK1, JAK2, JAK3, and TYK2), and inhibition of different groups of these kinases is being investigated in vitiligo and multiple other diseases.3 Binding of IFN-γ to the IFN-γ cell-surface receptor (IFNGR) creates a heterodimeric protein complex that initiates gene transcription through JAKs. As such, inhibition of either JAK1 or JAK2, through which IFNGR signals, decreases IFN-γ–mediated signaling. JAK inhibitors have been proposed to lead to repigmentation in vitiligo by disrupting IFN-γ and IL-15, a cytokine involved in maintenance of autoreactive tissue-resident memory T cells that are believed to contribute to vitiligo relapse after treatment cessation.3 JAK inhibitors may also improve vitiligo by enriching pathways that activate the hair follicle melanocyte stem cells.27
RUXOLITINIB
Ruxolitinib, which was previously approved for treatment of atopic dermatitis, received approval for nonsegmental vitiligo based on 52-week results from the phase 3 TRuE-V clinical trial program, which had built upon previous data from case studies and phase 2 trials.8,28 Findings from a case study showed rapid reversal of vitiligo and alopecia areata (a condition that causes hair loss) in a 35-year-old man treated with oral ruxolitinib, although the repigmentation was not maintained after treatment discontinuation.29 Additionally, after ruxolitinib treatment, the patient had a decrease in his serum CXCL10 level after experiencing an elevation for over 1 year, suggesting that targeting signaling mediated by IFN-γ and CXCL10 may be an effective strategy for treating vitiligo.29
A phase 2, open-label, proof-of-concept trial (NCT02809976) tested topical ruxolitinib 1.5% cream, which offers less toxicity risk than does systemic use, in patients with vitiligo affecting at least 1% of their total BSA.30,31 After 20 weeks of use, the mean percent improvement in overall VASI score was 23% (95% CI, 4%-43%; P = .02), with a response reported in 8 of 11 patients and the most notable responses occurring in the face. Adverse events (AEs) included erythema over the affected lesion in 8 of 11 patients, hyperpigmentation around the vitiligo patches on the facial and acral regions in 9 of 11 patients, and temporary papular eruptions or exacerbation of acne after facial application in 2 of 11 patients. However, no severe or persistent AEs were observed.30
To further study the efficacy and safety of ruxolitinib cream for patients with vitiligo, a randomized, dose-ranging, phase 2 study (NCT03099304) that spanned 156 weeks was performed.16 In the first part of the study, patients were randomly assigned 1:1:1:1:1 to receive ruxolitinib cream 1.5% twice daily, 1.5% once daily, 0.5% once daily, 0.15% once daily, or vehicle twice daily for 24 weeks. In the second part of the study, patients who had received either treatment with ruxolitinib cream 0.15% once daily or the vehicle and did not have at least a 25% improvement in facial VASI25 (F-VASI25) score were randomly assigned to receive a higher dose of 0.5% once daily, 1.5% once daily, or 1.5% twice daily for another 28 weeks. In the third part of the study, patients were able to receive open-label ruxolitinib cream 1.5% twice daily for another 104 weeks along with optional NBUVB phototherapy.16
The primary end point of facial VASI50 (F-VASI50) at week 24 was reached by a significantly higher percentage of patients treated with 1.5% twice daily (45% [OR, 24.7; 95% CI, 3.3-1121.4; P = .0001]) and 1.5% once daily
(50% [OR, 28.5; 95% CI, 3.7-1305; P < .0001]) than by those given the vehicle control. Additionally, more patients who received 0.5% once daily (26%) and 0.15% once daily (32%) achieved the primary end point than did those given the vehicle control (3%).16
At week 52, 58% of patients in the 1.5% twice daily group achieved F-VASI50. Serum concentrations of CXCL10 also decreased significantly from baseline in the group receiving the 1.5% twice daily dose, with reductions of 20% (P = .0011) reported at 24 weeks and 22% (P = .0006) noted at 52 weeks. All treatment-related AEs were of grade 1 or 2, with application-site pruritis being the most commonly reported event among patients who received ruxolitinib cream (1.5% twice daily, 3%; 1.5% once daily, 10%; 0.5% once daily, 10%; 0.15% once daily, 19%). Acne was also reported in 10% of patients who received ruxolitinib cream and 3% of those given vehicle. The authors concluded that ruxolitinib cream is an effective monotherapy for patients with vitiligo, with the dose of 1.5% twice daily yielding the best responses in F-VASI50.16
Based on the promising efficacy observed in previous studies, the TRuE-V clinical trial program studied the use of ruxolitinib cream in a large population of patients with vitiligo. This program included the phase 3 TRuE-V1 (NCT04052425) and TRuE-V2 (NCT04057573) trials, which assessed the safety and efficacy of ruxolitinib cream in patients with nonsegmental vitiligo.32 In each trial, approximately 300 patients (age, ≥ 12 years) with nonsegmental vitiligo were randomly assigned in a double-blind fashion to receive ruxolitinib cream 1.5% twice daily or vehicle control for 24 weeks. After this period, patients were able to receive another 28 weeks of treatment with ruxolitinib cream 1.5% if they passed the assessments conducted at baseline and at 24 weeks. For both studies, the primary end point was the proportion of patients who achieved a facial VASI75 (F-VASI75) at week 24. Key secondary end points included the proportion of patients with a F-VASI50 at week 24, a F-VASI75 at week 52, a facial VASI90 (F-VASI90) at week 24 and week 52, a total body VASI50 (T-VASI50) at week 24 and week 52, and a total body VASI75 (T-VASI75) at week 52. In addition, secondary end points included the proportion of patients with a vitiligo noticeability scale score of 4 or 5 at week 24 and percent change in facial BSA from baseline at week 24. The frequency, duration, and severity of AEs associated with ruxolitinib cream were also assessed.32
On October 2, 2021, the full 24-week results from the TRuE-V1 and TRuE-V2 studies were presented at the European Academy of Dermatology and Venereology 30th Congress and showed that, compared with vehicle control, more patients treated with ruxolitinib cream 1.5% twice daily achieved the primary and key secondary end points in both studies.33 At week 24, 51%, 29.9%, and 15% of patients who received ruxolitinib cream achieved F-VASI50, F-VASI75, and F-VASI90, respectively.33 Additionally, ruxolitinib cream was associated with a higher achievement of total body VASI50 than vehicle and a considerable improvement in the percent change in facial BSA from baseline.33 The safety and tolerability profile of ruxolitinib was similar to that reported in previous studies, and the therapy was not associated with any serious AEs.33
The 52-week results of the TRuE-V1 and TRuE-V2 studies, presented at the 2022 Academy of Dermatology Annual Meeting, included the double-blind and treatment extension periods and built on the outcomes achieved at 24 weeks. Approximately 75% of patients achieved a F-VASI50, approximately 50% achieved a F-VASI75, and approximately 30% achieved a F-VASI90. Notably, the F-VASI50 outcomes were consistent across both phase 2 and phase 3 clinical trials.34 Similar to week 24, at week 52, use of ruxolitinib cream was associated with improvements with T-VASI50 and facial BSA and did not result in serious AEs.34Based on these results, the FDA approved ruxolitinib for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.8,28
TOFACITINIB
Although inhibition of JAK1/2 for treatment of vitiligo is logical given its role in blocking IFN-γ signaling, targeting of other inflammatory and autoimmune pathways also may be important for effective and durable treatment.3 Important cytokine signaling through the common γ chain relies on JAK3, and cytokine signaling through the interferon-α/β receptor, IL-12 receptor, and IL-23 receptor requires TYK2. These signaling pathways have been involved in autoimmune and autoinflammatory conditions, but they have not been specifically linked to vitiligo. Efficacy associated with the therapeutic targeting of these pathways using small-molecule inhibitors may be due partially to unselective inhibition of multiple pathways with treatment.3
Five months of treatment with 5 mg/d of tofacitinib, a JAK1/3 inhibitor, led to nearly complete repigmentation of the face and hands in a case study of a woman in her 50s who had extensive vitiligo that had progressed over the previous year.35 In a retrospective case series of 10 consecutive patients with vitiligo treated with tofacitinib 5 to 10 mg given once or twice daily for an average of 9.9 months, 5 patients achieved some repigmentation (mean decrease in BSA involvement, 5.4%); however, repigmentation was achieved by 3 patients only on skin areas that had been exposed to the sun, 1 patient only with tofacitinib combined with full-body NBUVB phototherapy, and 1 patient on the dorsal sides of the hand after tofacitinib was combined with hand NBUVB phototherapy.9 This suggested that phototherapy may stimulate melanocytes as tofacitinib exerts anti-inflammatory effects; both components are needed to repigment the skin.9
Similarly, results from a pilot report of a small series of patients with facial vitiligo showed that treatment with tofacitinib cream 2% applied twice daily with concurrent NBUVB therapy 3 times per week for 2 to 4 months led to a mean improvement of 70% (range, 50%-87%) in F-VASI, with “good” to “excellent” repigmentation in all 11 patients.10 This suggested that topical tofacitinib and phototherapy have a synergistic relationship when used concurrently; however, additional controlled studies with larger sample sizes and long-term follow-up are needed.10
A COLLABORATIVE CARE APPROACH TO MANAGEMENT
In addition to improved therapeutic options, a collaborative care approach that includes psychosocial support may help improve QOL and optimize outcomes from treatment.36,37 A study that involved 8 patients with vitiligo and moderate-to-severe psychosocial problems who first completed 5 weekly sessions based on cognitive behavioral therapy resulted in a reduced DLQI score among all patients. In all, 7 patients maintained the reduction after completing a 7-week follow-up.36 Findings from another study showed a significant reduction in mean DLQI scores among patients with vitiligo after 20 days of climatotherapy; these same patients were able to sustain this improvement 1 year later (P < .05).37
Patient Education and Support
Patient education on the process of disease and repigmentation, which can take several months to show visible changes, is important to support adherence to treatment.18 Patients should understand that repigmentation is most successful when the treatment is used as prescribed and that success is influenced by disease duration, lesion distribution, and percentage of affected BSA. Patients may start to see results by 3 months but typically need to receive treatment for 2 or more years for optimal outcomes.38 Patients may benefit from connecting with organizations such as the Global Vitiligo Foundation, which offers patient education, support groups, research, and additional advocacy resources to help improve QOL for patients with vitiligo.39
Technology
Methods such as digital image techniques may assist providers and study investigators in objectively measuring repigmentation and response to therapy.18 One study used principal component analysis to decrease the dimensions of digital skin images from 3 to 2 principal components and then used independent component analysis to represent areas of skin in terms of melanin and hemoglobin composition.18 The researchers used a thresholding method based on the Euclidean distance to segment areas of vitiligo in the melanin images. A repigmentation percentage was used to describe the difference between pre- and post-treated images of the skin. The researchers used this imaging technology on 20 patients with vitiligo (generalized, acrofacial, segmental, focal, and universal) and detected repigmentation of lesion areas after 6 weeks of treatment. Additionally, the repigmentation percentages measured using the experimental method were consistent with PGA range and did not yield errors. The authors concluded that the digital imaging technology and analysis method may be able to accurately identify vitiligo areas and progression of repigmentation sooner than can the PGA system typically used.18
LOOKING AHEAD
Much remains to be learned about the initiation and progression of vitiligo, yet recent findings regarding the pathogenesis of this skin condition are likely to inform future treatments that are safer and more targeted and effective than are currently available options.3 The JAK 1/2 inhibitor ruxolitinib, targeting the signaling axis involving IFN-y and related chemokines, is the first approved medical therapy for vitiligo.3,8 Early-phase trials of other combined JAK inhibitors also are ongoing, including a phase 2 randomized trial (NCT03468855) investigating the safety, tolerability, and efficacy of using a 0.46% topical solution of ATI-50002 (a JAK1/3 inhibitor) in patients with nonsegmental facial vitiligo. In addition, a phase 2b, randomized, double-blind, parallel group, multicenter study (NCT03715829) is studying the efficacy and safety of PF-06651600 (a JAK3 inhibitor); this study includes an extension period to study the efficacy and safety of PF-06651600 and PF-06700841 (a JAK1/TYK2 inhibitor) in patients with active nonsegmental vitiligo.3,40,41
Finding ways to target the tissue-resident memory T cells involved in disease relapse after discontinuation of treatment (such as through IL-15) may improve long-term effects of therapy.3 Additionally, reestablishing homeostasis in the skin may be an alternative approach for inhibiting inflammation, which could be accomplished by activating regulatory T cells or using treatments that lead to immune privilege in the hair follicle. Furthermore, enhancing regeneration of melanocyte stem cells may have a synergistic effect with immune therapies and provide a more convenient alternative to phototherapy.3
Vitiligo and other autoimmune diseases have similar pathogenic mechanisms, and research in 1 autoimmune disease area is likely to lead to gains in knowledge about others.3 Thus, more research is needed to improve knowledge about vitiligo and similar autoimmune-related disorders.3
REFERENCES
- Niu C, Aisa HA. Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules. 2017;22(8):1303. doi:10.3390/molecules22081303
- Bowcock AM, Fernandez-Vina M. Targeting skin: vitiligo and autoimmunity. J Invest Dermatol. 2012;132(1):13-15. doi:10.1038/jid.2011.353
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648. doi:10.1146/annurev-immunol-100919-023531
- Taïeb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360(2):160-169. doi:10.1056/NEJMcp0804388
- Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006;72(4):315-321. doi:10.4103/0378-6323.26722
- Sheth VM, Guo Y, Qureshi AA. Comorbidities associated with vitiligo: a ten-year retrospective study. Dermatology. 2013;227(4):311-315. doi:10.1159/000354607
- Birlea SA, Goldstein NB, Norris DA. Repigmentation through melanocyte regeneration in vitiligo. Dermatol Clin. 2017;35(2):205-218. doi:10.1016/j.det.2016.11.015
- Incyte announces U.S. FDA approval of Opzelura (ruxolitinib) cream for the treatment of vitiligo. Incyte. July 18, 2022. Accessed July 19, 2022. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-us-fda-approval-opzeluratm-ruxolitinib-cream-0
- Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77(4):675-682.e1. doi:10.1016/j.jaad.2017.05.043
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81(2):646-648. doi:10.1016/j.jaad.2019.04.032
- Salzes C, Abadie S, Seneschal J, et al. The vitiligo impact patient scale (VIPs): development and validation of a vitiligo burden assessment tool. J Invest Dermatol. 2016;136(1):52-58. doi:10.1038/JID.2015.398
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149(2):159-164. doi:10.1001/jamadermatol.2013.927
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. doi:10.1111/j.1365-2230.1994.tb01167.x
- Speeckaert R, Lambert J, Bulat V, Belpaire A, Speeckaert M, van Geel N. Autoimmunity in segmental vitiligo. Front Immunol. 2020;11:568447.
doi:10.3389/fimmu.2020.568447 - Chang WL, Lee WR, Kuo YC, Huang YH. Vitiligo: An autoimmune skin disease and its immunomodulatory therapeutic intervention. Front Cell Dev Biol. 2021;9:797026. doi:10.3389/fcell.2021.797026
- Rosmarin D, Pandya AG, Lebwohl M, et al Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110-120. doi:10.1016/S0140-6736(20)30609-7
- Wang XX, Wang QQ, Wu JQ, et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;174(6):1318-1326. doi:10.1111/bjd.14416
- Fadzil MHA, Norashikin S, Suraiya HH, Nugroho H. Independent component analysis for assessing therapeutic response in vitiligo skin disorder. J Med Eng Technol. 2009;33(2):101-109. doi:10.1080/03091900802454459
- Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo. Meta-analysis of the literature. Arch Dermatol. 1998;134(12):1532-1540. doi:10.1001/archderm.134.12.1532
- Bhatnagar A, Kanwar AJ, Parsad D, De D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. J Eur Acad Dermatol Venereol. 2007;21(5):638-642. doi:10.1111/j.1468-3083.2006.02035.x
- Yones SS, Palmer RA, Garibaldinos TM, Hawk JLM. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs narrowband-UV-B therapy. Arch Dermatol. 2007;143(5):578-584. doi:10.1001/archderm.143.5.578 Published correction appears in Arch Dermatol. 2007;143(7):906.
- Hann SK, Kim HI, Im S, Park YK, Cui J, Bystryn JC. The change of melanocyte cytotoxicity after systemic steroid treatment in vitiligo patients. J Dermatol Sci. 1993;6(3):201-205. doi:10.1016/0923-1811(93)90039-r
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2015;24(2):CD003263. doi:10.1002/14651858.CD003263.pub5
- Dang YP, Li Q, Shi F, Yuan XY, Liu W. Effect of topical calcineurin inhibitors as monotherapy or combined with phototherapy for vitiligo treatment: a meta-analysis. Dermatol Ther. 2016;29(2):126-133. doi:10.1111/dth.12295
- Wong R, Lin AN. Efficacy of topical calcineurin inhibitors in vitiligo. Int J Dermatol. 2013;52(4):491-496. doi:10.1111/j.1365-4632.2012.05697.x
- Silpa-Archa N, Griffith JL, Huggins RH, et al. Long-term follow-up of patients undergoing autologous noncultured melanocyte-keratinocyte transplantation for vitiligo and other leukodermas. J Am Acad Dermatol. 2017. doi:10.1016/j.jaad.2017.01.056
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1(9):e1500973.
doi:10.1126/sciadv.1500973 - Opelzura. Prescribing information. Incyte Dermatology; 2021. Accessed March 21, 2022. https://www.opzelura.com/prescribing-information.pdf
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74(2):370-371. doi:10.1016/j.jaad.2015.09.073
- Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. 2017;76(6):1054-1060.e1. doi:10.1016/j.jaad.2017.02.049
- Topical ruxolitinib for the treatment of vitiligo. Clinicaltrials.gov. Updated August 31, 2020. Accessed April 4, 2022. https://clinicaltrials.gov/ct2/show/NCT02809976
- Incyte announces acceptance and priority review of sNDA for ruxolitinib cream (Opzelura) as a treatment for patients with vitiligo. Incyte. December 14, 2021. Accessed February 24, 2022. https://s21.q4cdn.com/114423841/files/doc_news/Incyte-Announces-Acceptance-and-Priority-Review-of-sNDA-for-Ruxolitinib-Cream-Opzelura-as-a-Treatment-for-Patients-with-Vitiligo-2021.pdf
- Incyte announces full results from phase 3 TRuE-V program evaluating ruxolitinib cream (Opzelura) in patients with vitiligo. Incyte. October 2, 2021. Accessed February 24, 2022. https://investor.incyte.com/press-releases/press-releases/2021/Incyte-Announces-Full-Results-From-Phase-3-TRuE-V-Program-Evaluating-Ruxolitinib-Cream-Opzelura-in-Patients-With-Vitiligo/default.aspx
- Incyte announces 52-week data from the phase 3 TRuE-V program evaluating ruxolitinib cream (Opzelura™) in patients with vitiligo. Business Wire. March 26, 2022. Accessed May 25, 2022. https://www.businesswire.com/news/home/20220326005005/en/Incyte-Announces-52-Week-Data-From-the-Phase-3-TRuE-V-Program-Evaluating-Ruxolitinib-Cream-Opzelura%E2%84%A2-in-Patients-With-Vitiligo
- Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110-1112. doi:10.1001/jamadermatol.2015.1520
- Jha A, Mehta M, Khaitan BK, Sharma VK, Ramam M. Cognitive behavior therapy for psychosocial stress in vitiligo. Indian J Dermatol Venereol Leprol. 2016;82(3):308-310. doi:10.4103/0378-6323.175925
- Krüger C, Smythe JW, Spencer JD, et al. Significant immediate and long-term improvement in quality of life and disease coping in patients with vitiligo after group climatotherapy at the Dead Sea. Acta Derm Venereol. 2011;91(2):152-159. doi:10.2340/00015555-1037
- Treatments. Global Vitiligo Foundation. Accessed May 17, 2022. https://globalvitiligofoundation.org/treatments/
- About us. Global Vitiligo Foundation. Accessed May 17, 2022. https://globalvitiligofoundation.org/about-us/
- A study of ATI-50002 topical solution for the treatment of vitiligo. Clinicaltrials.gov. Updated November 30, 2020. Accessed April 5, 2022. https://clinicaltrials.gov/ct2/show/NCT03468855
- A phase 2b study to evaluate the efficacy and safety profile of PF-06651600 and PF-06700841 in active non-segmental vitiligo subjects. Clinicaltrials.gov. Updated March 25, 2022. Accessed April 5, 2022. https://clinicaltrials.gov/ct2/show/NCT03715829
