Increased Mortality Among Patients Taking Digoxin

Timir K. Paul, MD, PhD, MPH
Review

The landmark Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial enrolled 4060 atrial fibrillation (AF) patients who were at high risk of stroke or death. The patients were randomized to a rate control (n = 2070) versus rhythm control (n = 2033) strategy, with a mean follow up of 3.5 years and maximum of 6 years. The current analysis by Whitbeck et al was a post hoc analysis from AFFIRM data that used propensity scores, a multivariate Cox proportional hazards model, and sensitivity analyses. Mortality end points in patients with and without digoxin were compared. Analyses were performed in all patients—separately in men and women and in subgroups based on the presence or absence of heart failure (HF) and/or ejection fraction <40% after controlling for comorbidities and clinical variables associated with mortality.
Overall, 69.4% of patients received digoxin within 6 months of randomization, during the study, or both. A total of 39.3% of the study cohort was female and 26.5% had HF. Among the patients who died, 56.3% were taking digoxin at their last follow-up visit. After adjustment for clinical risk factors, comorbidities, and propensity scores, digoxin was independently associated with a 41% increase in all-cause mortality (P <0.001), 35% higher risk of deaths from cardiovascular causes (P = 0.016), and 61% increase in arrhythmic mortality (P = 0.009) The all-cause mortality remained higher with use of digoxin among patients with and without HF (estimated hazard ratio [EHR], 1.41 [P = 0.010] and 1.37 [P = 0.019], respectively). There was no significant digoxingender interaction noted and the increase in all-cause mortality was observed in both men and women.
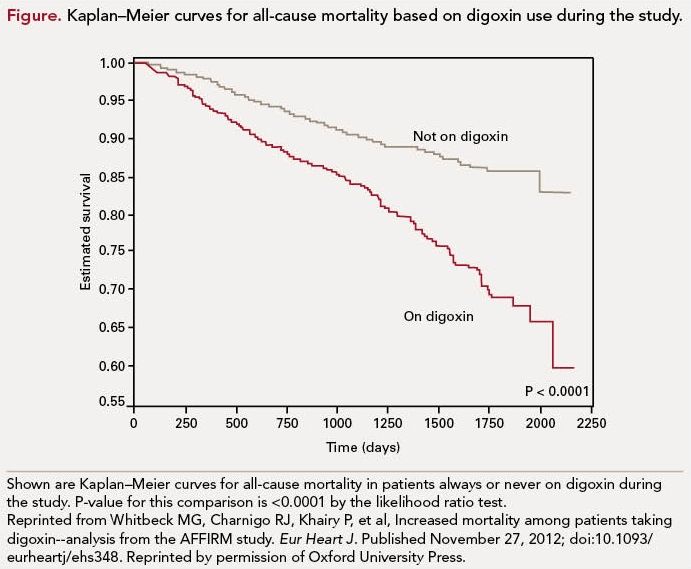
The authors concluded that use of digoxin in AF patients significantly increased allcause mortality independent of gender and the presence or absence of HF.
References
1. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:523- 533.
2. Hallberg P, Lindback J, Lindahl B, et al. Digoxin and mortality in atrial fibrillation: a prospective cohort study. Eur J Clin Pharmacol. 2007;63:959- 971.
3. Wyse DG, Waldo AL, DiMarco JP, et al; AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
4. Winkelmayer WC, Kurth T. Propensity scores: help or hype? Nephrol Dial Transplant. 2004; 19:1671-1673.
5. Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity and in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497-504.
6. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871-878.
7. Ahmed A, Pitt A, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity matched study of the DIG trial. Int J Cardiol. 2008;123:138-146.
8. Digitalis Investigation Group, Ahmed A, Waagstein F, Pitt B, et al. Effectiveness of digoxin in reducing one-year mortality in chronic heart failure in the Digitalis Investigation Group trial. Am J Cardiol. 2009;103:82-87.
Commentary Digoxin Safety Concerns Raised in AF Patients
This study demonstrated that use of digoxin in AF patients significantly increases all-cause mortality regardless of gender and the presence or absence of HF, and raises important safety concerns for the use of digoxin in patients with AF.
Prospective randomized, controlled trials were conducted on digoxin in patients with HF, excluding AF patients.1 Although the Digitalis Investigation Group (DIG) trial1 showed digoxin had a neutral effect on mortality in patients with HF and without AF, Hallberg and colleagues’ Swedish cohort study showed a 42% increased risk of mortality in patients with AF and without HF.2
By assessing baseline characteristics in the digoxin versus no-digoxin group, it seems that the digoxin group consists of a sicker patient population. Significantly higher numbers of patients with cardiomyopathy, valvular heart disease, pulmonary disease, symptoms during AF, and antiarrhythmic drug failure were found in the digoxin group. ß-blockers have proven mortality benefit and more patients were on ß-blockers who were not taking digoxin (34% vs 26%, P<0.0001). There were no baseline data on age, gender, and diabetes in both groups.

There were several limitations of this study. First of all, this was a post hoc analysis. The major limitation of this study was that there was no documentation on digoxin dose, serum digoxin levels, or the exact duration and/or adherence to digoxin treatment. There was no specific recommendation on digoxin dose in the AFFIRM protocol3 and data on renal function were not available.
Although propensity scores were used to make a balance between 2 groups, this score did not include age, gender, diabetes, and angiotensin-converting enzyme inhibitors (ACEIs) as covariates. AF is a disease of older age; diabetes is considered a risk factor for stroke in AF, and ACEI has a mortality benefit in HF patients. Further, propensity score analysis does not take into account unknown potential confounders.4
In the AFFIRM3 trial strict heart rate control was the target and higher doses of digoxin may have been used in combination with ß-blockers or calcium channel blockers (CCBs). Digoxin was also utilized as a sole agent for rate control in 17% of patients and there was a high likelihood that higher digoxin doses may have been used for stringent rate control in these patients. Toxicity may have occurred with this higher dose of digoxin as high serum level of digoxin (>1.0 ng/mL) was the goal in the AFFIRM trial. Studies demonstrated that the higher doses may predispose to arrhythmia and increase mortality,5,6 and low serum digoxin concentration (0.5-0.9 ng/mL) reduces mortality.7,8
Proper selection of patients, low serum digoxin level, and close monitoring of electrolytes (such as hypokalemia, hypomagnesemia) and renal function would decrease potential toxicity and subsequent mortality. Most patients with HF take furosemide and/or hydrochlorothiazide, and hypokalemia is common in these patient populations. The concomitant use of other drugs such as verapamil, amiodarone, spironolactone, and flecainide may increase serum digoxin and cause digoxin toxicity.
Based on this study’s findings, physicians should use ß-blockers or CCBs as a first-line therapy to control optimal heart rate in patients with AF. The current American College of Cardiology/American Heart Association guidelines do not recommend digoxin as a first-line therapy or sole agent for rate control in AF. This study does not provide a new recommendation, but it suggests that digoxin should be used with caution in patients with AF. If digoxin is required for additional rate control as a combination therapy, I recommend using lower doses and careful clinical follow-up and monitoring of digoxin level, at least for the initial period of treatment. Potential drug interactions should be considered when starting new medications or concurrent use of medications (eg, amiodarone, verapamil, etc). Strict monitoring of digoxin levels and adjustment of digoxin dose accordingly to keep levels between 0.5 ng/mL and 0.9 ng/mL is key to avoiding toxicity. Patients should be aware of potential adverse effects and seek prompt attention from their physicians if they experience nausea, palpitations, or syncope, as these may predispose to arrhythmic death.
Based on the results of this study, I would not completely withdraw digoxin for treating AF but would use it cautiously. Digoxin is effective and is very useful in certain clinical situations such as patients with hypotension and severe chronic obstructive pulmonary disease/asthma who would not tolerate ß-blockers or CCBs and/or patients who continue to have symptoms despite optimal doses of these AV nodal blockers.
Low-dose, close monitoring of digoxin level and clinical symptoms is key. It is difficult to ignore the magnitude of the effect of digoxin on overall mortality in this study. However, I would not completely disregard digoxin in the treatment of AF patients based on these results from post hoc analyzed data. My own approach would be conservative, taking into account that while digoxin appears to increase mortality in AF patients, until future randomized trials or multiple observational studies show similar results, I would not eliminate the use of digoxin in this population, particularly in cases of low serum digoxin levels. To sum up, less is more in digoxin therapy, in either isolated AF or patients with AF and HF. “
About the Author
Timir K. Paul, MD, PhD, MPH, is Assistant Professor, Division of Cardiology, Department of Medicine, and Director of Preventive Medicine and Cardiac Rehabilitation at Quillen College of Medicine, East Tennessee State University, Johnson City, TN. He received his MD from Dhaka Medical College, University of Dhaka, Dhaka, Bangladesh, and his PhD and MPH degrees from Tulane University. Dr Paul did his fellowship in cardiology at Ochsner Clinic, New Orleans, LA, and in interventional cardiology at the University of North Carolina, Chapel Hill. He has published many original research articles and reviews.
Whitbeck MG, Charnigo RK, Khairy P, et al. Increased mortality among patients taking digoxin— analysis from the AFFIRM study [published online November 27, 2012]. Eur Heart J. doi:10.1093/ eurheartj/ehs348.
