Real-World Data Demonstrate Safety, Efficacy of Upadacitinib for PsA
Achievement of minimal disease activity and remission were observed in approximately half of patients with psoriatic arthritis receiving upadacitinib.
Michele Maria Luchetti Gentiloni, MD
Credit: Loop

In addition to confirming the efficacy and safety of upadacitinib treatment in patients with psoriatic arthritis (PsA), findings published in Arthritis Research and Therapy identified the predictors of minimal disease activity (MDA) response to treatment at the 6-month mark.1
“Despite the available therapies, only approximately one-third of patients achieve and maintain minimal disease activity,” wrote lead investigator Michele Maria Luchetti Gentiloni, MD, affiliated with the Department of Molecular and Biological Sciences, Marche Polytechnic University, and Department of Internal Medicine, Azienda Ospedaliero Universitaria delle Marche, Ancona, Italy, and colleagues. “Thus, there is a significant need for novel therapies effective on as many disease domains as possible, providing personalized treatment for each patient’s phenotypes.”
Tofacitinib and upadacitinib have been recently approved for PsA treatment. In the SELECT 1 and SELECT 2 trials, upadacitinib demonstrated significant improvements in pain, fatigue, and quality of life.2
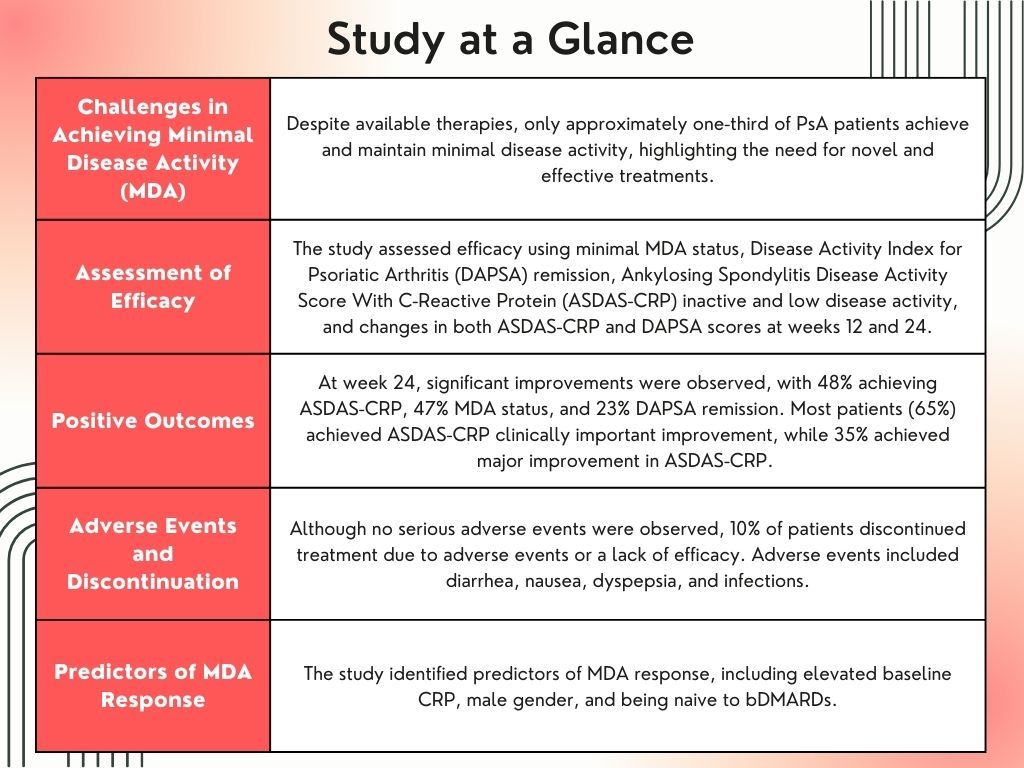
A total of 126 patients with PsA treated with upadacitinib, a selective Janus kinase (JAK) inhibitor, were included in the observational study conducted at 10 Italian centers. Efficacy was determined by the proportion of patients with MDA status, low disease activity (LDA), Disease Activity Index for Psoriatic Arthritis (DAPSA) remission, Ankylosing Spondylitis Disease Activity Score With C-Reactive Protein (ASDAS-CRP) inactive and low disease activity, and change from baseline in both ASDAS-CRP and DAPSA scores, which were assessed at week 12 and week 24. Other endpoints were the proportion of DAPSA minor, moderate, and major improvement, and ASDAS major improvement (MI) and clinically important improvement (CII). Treatment-related adverse events were monitored throughout the study. Demographic data, such as age, sex, and body mass index (BMI), as well as disease information, including duration, previous treatments, and comorbidities, were collected. A multivariate analysis assessed clinical predictors of MDA response at week 24.
At baseline 98% (n = 124) of patients reported peripheral involvement and 43% (n = 54) had axial involvement. Most (87%, n = 110) were intolerant or resistant to treatment with biologic disease-modifying antirheumatic drugs (bDMARDs).
The mean change from baseline for DAPSA was 15.9 ± 13.5 (P <.001) and 1.21 ± 0.97 (P <.001) for ASDAS-CRP. At week 24, ASDAS-CRP, MDA status, and DAPSA remission were observed in 48%, 47%, and 23%, respectively. Most (65%) patients achieved ASDAS-CRP CII, while 35% achieved ASDAS-CRP MI. Minor, moderate, and major improvement in DAPSA was reported in 67%, 39%, and 23%, respectively.
Although no serious adverse events were observed, 10% (n = 13) of patients discontinued treatment due to adverse events or a lack of efficacy. Seventeen patients reported 21 adverse events, with the most common being diarrhea, nausea, dyspepsia, and infections.
MDA response was linked to an elevated baseline CRP (odds ratio [OR] 2.49, 95% confidence interval [CI] 1.02 – 6.12, P = .046), male gender (OR 2.54, 95% CI 1.03 – 6.25 P = .043), and being naïve to bDMARDs (OR 4.13, 95% CI 1.34–12.71, P = .013).
Investigators regarded the observational nature of the study as a limitation. They also noted results may not be fully representative of the entire spectrum of PsA. As the study is ongoing, the short-term observation period may have led to an underestimation of discontinuation or the incidence of adverse events. Finally, some confounding factors, including the dose of methotrexate or the use of nonsteroidal anti-inflammatory drugs, were not included in the analysis.
“The 24-week results from the UPREAL-PsA study confirm the remarkable efficacy and good safety profile of upadacitinib therapy in real-life PsA, with the higher efficacy of upadacitinib demonstrated in males, bio-naïve patients, and those with elevated baseline CRP,” investigators concluded.
References
- Luchetti Gentiloni MM, Paci V, Carletto A, et al. Upadacitinib effectiveness and factors associated with minimal disease activity achievement in patients with psoriatic arthritis: preliminary data of a real-life multicenter study. Arthritis Res Ther. 2023;25(1):196. Published 2023 Oct 11. doi:10.1186/s13075-023-03182-9
- McInnes IB, Kato K, Magrey M, Merola JF, Kishimoto M, Pacheco-Tena C, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7:e001838.