Single- Versus Dual-Chambered ICDs for Primary Prevention: Association with Mortality, Readmissions, and Complications
Suneet Mittal, MD, FACC, FHRS
Review

Based on the results of numerous randomized clinical trials, the implantable cardioverter defibrillator (ICD) is indicated for the primary prevention of sudden cardiac death in high-risk patients.1 Although clinical trials have exclusively utilized a singlechamber ICD system, dual-chamber systems incorporating an additional atrial lead remain a popular choice with implanting physicians. 2 Even when the patient lacks an indication for atrial pacing, the atrial lead can provide information about the presence of atrial tachyarrhythmias and assist with rhythm (supraventricular versus ventricular) discrimination. However, the need to implant an additional lead may be associated with increased procedural complications that may translate into worse short- and long-term outcome. Thus, the optimal system remains unknown.
Study Details
Peterson and colleagues evaluated data from the National Cardiovascular Data Registry’s (NCDRs) ICD registry, which was established in 2005 by the Centers for Medicare & Medicaid Services to capture information about clinical, demographic, and procedural characteristics, along with pre-hospital discharge complications in Medicare beneficiaries undergoing ICD implantation for a primary prevention indication.3 The authors identified 32,034 patients who met the primary inclusion criteria: primary prevention ICD implant, a left ventricular ejection fraction ≤35%, no requirement for cardiac resynchronization therapy (CRT), and the absence of an indication for atrial pacing. Of these patients, 12,246 (38%) were implanted with a single-chamber device and 19,788 (62%) were implanted with a dual-chamber device.
Procedural complications that were ascertained included the following: within the first 30 days of the implant procedure, a pneumothorax requiring a chest tube, a hematoma requiring a blood transfusion, or surgical reintervention for evacuation, and/or cardiac tamponade; and within the first 90 days of the implant procedure, mechanical complication(s) requiring reoperation for system, generator, or lead revision, a device-related infection, and/or recurrent ICD implant. Long-term outcome was ascertained through assessment of all-cause mortality and rehospitalization for heart failure or for any reason within the first year of device implantation.
Compared with patients who received a single-chamber device, patients who received a dual-chamber device were more likely to be male, have a history of nonsustained or sustained ventricular tachycardia, have a history of syncope, have ischemic heart disease, have an ejection fraction of ≥30%, have first-degree atrioventricular (AV) delay, right or left bundle branch block, and have a wider QRS duration. Patients who received a dual-chamber device were much more likely to experience a mechanical complication as well as rehospitalization for heart failure.
The authors subsequently performed a propensity-matching analysis of 11,619 single-chamber ICD patients to 11,619 dual-chamber ICD patients. Standardized differences were less than 10% for all variables. In this analysis, single-chamber systems were associated with significantly lower rate of complications (3.51% vs 4.72%, P <0.001; risk difference, -1.20% [95% confidence interval (CI), -1.72 to -0.69]); the largest absolute difference was observed in the incidence of mechanical complications (1.43% vs 1.98%, P = 0.001; risk difference, -0.55% [95% CI, -0.88 to -0.22]). The highest complication rate (6.43%) was observed in women undergoing dual-chamber ICD implantation.
Commentary Why Are So Many Patients Receiving Dual-Chamber ICDs?
In patients with left ventricular dysfunction who do not meet criteria for cardiac resynchronization therapy (CRT), the implanting physician must choose between a single- and dual-chamber ICD system. In patients who need atrial pacing support, a dual-chamber ICD system will clearly be necessary. However, this is required in a minority of patients. For example, in the study by Peterson and colleagues, only 12,767 (28%) out of 44,801 patients undergoing ICD implantation had a documented indication for atrial pacing. Of the remaining 72% of patients, 62% received a dual-chamber ICD system.
The central question is, why are so many patients receiving dualchamber ICD systems? From the standpoint of an insurance carrier, single-chamber systems are preferable to dual-chamber systems because they are cheaper, have the greatest longevity, and are associated with the lowest procedural complication rates. From the standpoint of a hospital, the reimbursement is the same for singleor dual-chamber ICD systems despite the increased cost associated with dual-chamber systems (additional atrial lead, more expensive generator); thus, they too would prefer single-chamber ICD systems. From the standpoint of the physician, there is no additional reimbursement for the time and effort that it takes to add the atrial lead. Thus, for the patient, what are the possible advantages and disadvantages of the additional atrial lead?
The first advantage is that in the event that the patient develops an indication for atrial pacing, the patient will avoid a repeat surgical procedure to add the atrial lead and replace the ICD generator. Although reoperation is clearly desirable, it should be noted that randomized clinical trials have shown that only 7% of patients develop an indication for pacing.4 In some of these patients, the pacing indication is development of atrioventricular (AV) block; these patients will require reoperation anyway to add a left ventricular lead to provide CRT.
The second advantage is that the availability of the sensed atrial electrogram can help the device, as well as the physician, discriminate between supraventricular and ventricular arrhythmias. Unfortunately, although all device manufacturers incorporate a proprietary algorithm to differentiate supraventricular from ventricular arrhythmias, it has been difficult to demonstrate that these algorithms reduce the likelihood of inappropriate shocks. This is partly due to the deficiencies in the algorithms and partly due to the failure of physicians to routinely engage these algorithms during device programming. More recently, a randomized clinical trial has suggested that the best method to reduce inappropriate shocks and improve patient outcome is to program the ICD to treat ventricular arrhythmias only if their rate exceeds 200 bpm5; this finding greatly minimizes the need to rely on dedicated discrimination algorithms.
The third advantage is having information about the presence of atrial high-rate episodes (AHREs). Recent data suggest that AHREs lasting more than 6 minutes may identify patients at high risk for thromboembolism, especially in those with a CHADS2 score of 3 or greater.6 Since modern devices can be wirelessly coupled to a home monitoring system, physicians can be notified virtually immediately after the onset of an AHRE. This may afford the physician an opportunity to consider initiation of anticoagulation therapy to prevent the risk of stroke and other systemic thromboembolism in high-risk patients.
However, Peterson and colleagues have identified the clear disadvantages that counter these advantages. An approximate 1.25% excess in the absolute rate of complications was observed within the first 90 days of the procedure. This was driven largely by excess in the rate of pneumothorax requiring a chest tube, cardiac tamponade, and a mechanical complication requiring a system revision. In women, this excess risk increased by 1.75%. Finally, patients who received a single-chamber ICD were slightly less likely than their dual-chamber counterparts to require rehospitalization for heart failure within the first year of device implantation.
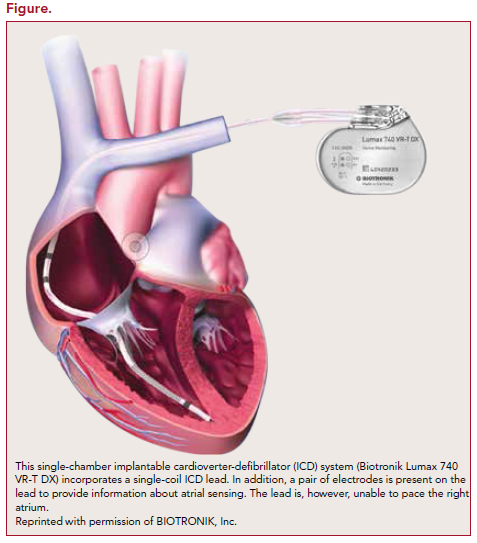
Recently, a new type of ICD system (Figure) has been introduced by Biotronik that may be able to provide many of the key advantages associated with the atrial lead while eliminating the complications inherent to adding a second intracardiac lead. This system uses an ICD lead that incorporates an additional pair of electrodes that provide information about atrial sensing. Although atrial pacing is not available, the ability to record a sensed atrial electrogram should facilitate rhythm discrimination and provide information about AHREs. Additional data are needed about the long-term performance of this lead.
In conclusion, Peterson and colleagues have once again shown us that thoughtful consideration should be given to what is being implanted in any given patient.
References
1. Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/ SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter- defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318-1368.
2. Dewland TA, Pelligrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2011;58:1007-1013.
3. Peterson PN, Varosy PD, Heidenreich PA, et al. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025-2034.
4. Sweeney MO, Ellenbogen KA, Tang ASL, et al; for the Managed Ventricular Pacing Versus VVI 40 Pacing Trial Investigators. Atrial pacing or ventricular backup-only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:1552-1560.
5. Moss AJ, Schuger C, Beck CA, et al; for the MADIT-RIT trial investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275-2283.
6. Healey JS, Connolly SJ, Gold MR, et al; for the ASSERT investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120-129.
About the Author
Suneet Mittal, MD, FACC, FHRS, is Director of Electrophysiology at The Valley Hospital Health System, in Ridgewood, NJ, and Associate Professor of Clinical Medicine at Columbia University, New York, NY. He received his MD from Boston University School of Medicine, and did an internship and residency at the Hospital of the University of Pennsylvania (HUP) in Philadelphia, PA, as well as fellowships in cardiology at HUP and in cardiac electrophysiology at The New York Hospital-Cornell University Medical Center. Dr. Mittal has written many articles and chapters on electrophysiology and is a frequent participant in national and international symposia, meetings, and scientific sessions. Dr. Mittal has served as a consultant to Biotronik, Boston Scientific, Medtronic, Sorin, and St. Jude Medical.
Peterson PN, Varosy PD, Heiden PA, et al. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025-2034.
