Upadacitinib: An Evolving Treatment for Ulcerative Colitis
The inhibition of Janus kinase to prevent downstream signaling of proinflammatory cytokines has been shown to be a novel method of treating ulcerative colitis.
Ravi Shah, MD

Despite the evolution of biologic therapy used to treat ulcerative colitis (UC) over the past few decades, there remains an unmet need of options to both induce and maintain remission of moderate to severe UC.
The inhibition of Janus kinase (JAK) to prevent downstream signaling of proinflammatory cytokines has been shown to be a novel method of treating UC.
Although the effectiveness of nonspecific JAK inhibition has been successful, concerns of the safety profile has limited its clinical use.
Upadacitinib (UPA) is a more selective JAK-1 inhibitor that was previously approved for use in rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and atopic dermatitis.
Danese and Vermeire et al. conducted a phase 3, multicenter, randomized, double-blind, placebo-controlled trial consisting of 2 replicate induction studies and a maintenance study assessing the efficacy and safety of UPA in patients with moderately to severely active UC who had either an inadequate or loss of response to 5-aminosalicylate (5-ASA), corticosteroids, immunomodulator, or biologic.
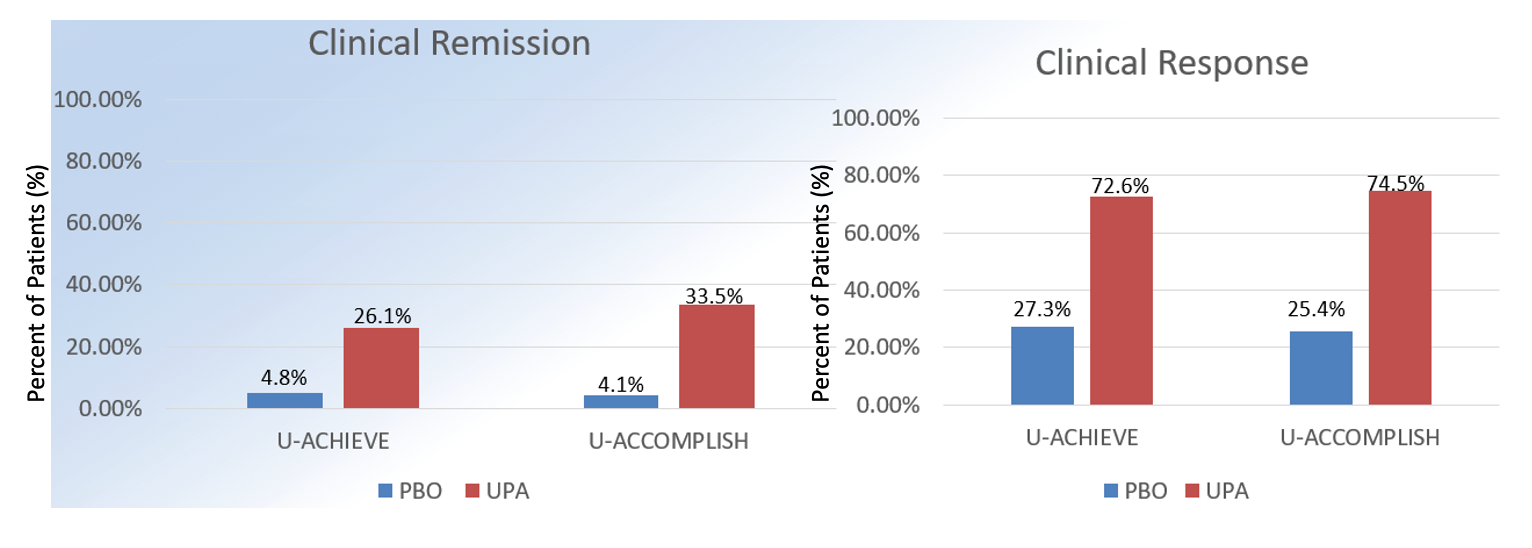
The 2 replicate induction studies randomized patients with moderately-severely active UC to UPA 45 mg orally daily versus placebo (n = 319 vs n = 154; n = 341 vs n = 174). Patients receiving UPA more successfully achieved the primary endpoint- clinical remission, defined as an adapted Mayo score at week 8, compared to those receiving placebo (26% vs 5%; 33% vs 4%; P <.001).
The UPA group also significantly achieved the secondary endpoints, including endoscopic improvement, endoscopic remission, clinical response, histologic improvement, no bowel urgency, no abdominal pain, and quality of life questionnaire scores. In a subgroup analysis of ~50% of patients with previous biologic failure, clinical remission was achieved more in UPA-treated compared to placebo-treated patients (17.9% vs 0%; 64.6% vs 12.8%). The results of the study are summarized in the tables.
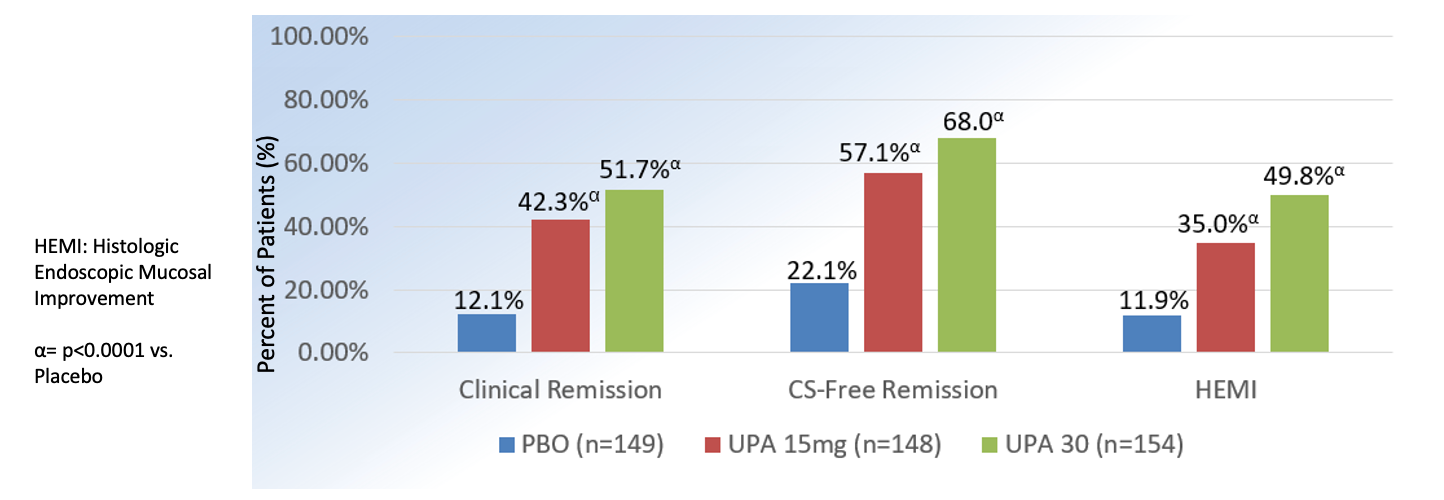
The maintenance study randomized patients who achieved clinical response from the two phase 3 induction studies and a phase 2b study to receive daily UPA 15 mg (n = 149), UPA 30 mg (n = 154) or placebo (n = 149) for 54 weeks.
Clinical remission at 52 weeks was more significantly achieved by patients treated with UPA 15 mg (42%) and UPA 30 mg (52%) compared to those who received placebo (12%; P <.001). Similar to the induction studies, UPA 15 mg and UPA 30 mg more significantly achieved the secondary endpoints compared to placebo. In a subgroup analysis of patients with prior biologic failure, clinical remission was achieved at higher rates in the UPA 15 mg (40.5%) and UPA 30 mg (49.1%) compared to placebo groups (7.5%). The results of the study is also summarized below in tables.
U-ACHIEVE and U-ACCOMPLISH: Results
Adapted from Danese S et al. The Lancet. 2022, (399);10341:2113-2128
Rates of adverse events in the induction studies between UPA 45 mg and placebo groups (56% vs 60%; 53% vs 40%), and in the maintenance study between UPA 15 mg, UPA 30 mg and placebo groups (78% and 79% vs 76%) were relatively similar.
In both the induction and maintenance studies, serious adverse events and adverse events leading to discontinuation of treatment were less frequent in the UPA 45 mg group compared to the placebo group. Specifically, no events of adjudicated MACE, VTE, or gastrointestinal perforation were reported in the UPA 45 mg-treated patients. In the maintenance groups, adjudicated MACE or gastrointestinal perforation were not reported, but there were 2 non-serious VTE reported in the UPA 30 mg treated group that were deemed to be unrelated to the study drug. Rates of serious infections were similar in the UPA and placebo groups.
Herpes zoster led to discontinuation of UPA 45 mg and UPA 30 mg in 2 of 9 reported cases. There were 3 cases of cytomegalovirus that did not lead to discontinuation of therapy. There were 2 non-melanoma skin cancers reported amongst the UPA 30 mg group. Other reported non-serious side effects included nasopharyngitis, acne, mild-moderate liver test abnormalities, and total cholesterol elevations in the UPA-treated groups.
U-ACHIEVE: Upadacitnib Maintenance
Adapted from Danese S et al. The Lancet. 2022, (399);10341:2113-2128
Upadacitinib, a more selective JAK-1 inhibitor, is a promising addition to the therapeutic armamentarium against UC especially in those who have previously failed or developed immunogenicity to biologic therapies.
It remains unclear whether the reported adverse events are drug or patient characteristic-specific, but preventative measures (i.e, vaccination) may dissipate some of the serious infectious risks reported in the UPA trials. Future studies assessing the positioning of UPA amongst available treatment options for UC and the long-term safety profile are needed to further solidify this promising therapy for patients suffering from moderate to severe UC.