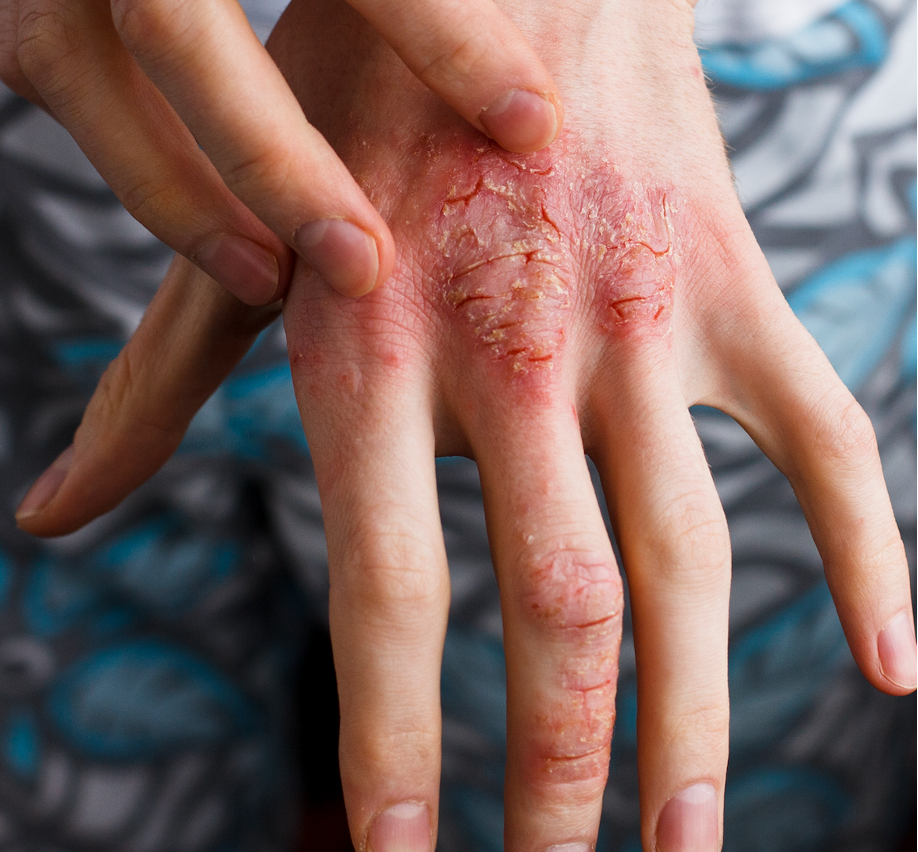
Switching to a Biosimilar from an Originator Deemed Safe and Effective in Psoriasis Treatment
Real-world evidence indicates that patients with psoriasis can safely and effectively undergo (non-medical) switching to a biosimilar.
Based on complementary real-world evidence, patients with psoriasis were able to safely and effectively switch to biosimilar therapies, specifically adalimumab, infliximab, and etanercept, according to the Journal of Dermatological Treatment.
“A barrier to the usage of biosimilars in clinical practice is a lack of confidence by physicians and patients,” investigators explained. “Both physicians and patients report preferences for reference products over biosimilars due to concerns for patient mental health, treatment efficacy, and patient safety. Provider hesitancy may stem from the concern that the development of biosimilars is not subject to the same scrutiny as that of novel biologics.”
Biosimilars offer a cost-effective alternative and increase treatment availability for patients. Investigators evaluated data from real-world studies in patients with psoriasis switching from biologics to biosimilars in this literature review, utilizing a PubMed and Google Scholar search. Key words included “switching between biosimilars in psoriasis,” “switching from adalimumab originator to biosimilar in psoriasis,” “switching from etanercept originator to biosimilar in psoriasis,” “switching from infliximab originator to biosimilar in psoriasis,” and “anti-tumor necrosis factor (TNF) inhibitor biosimilars in psoriasis.”
Ultimately, 7 articles on infliximab biosimilars, 6 articles on etanercept biosimilars, and 5 articles on biosimilars were included. The safety and efficacy profiles were comparable in those switching from the originator to biosimilars.
Among the 5 observational adalimumab studies, a similar efficacy pertaining to controlling cutaneous disease following the switch to biosimilar therapy was observed. Two of 3 studies analyzing articular disease showed no loss of disease control. An increase of mild adverse events (AEs) was reported in 2 of the 5 studies.
Observational etanercept biosimilar studies also observed efficacy regarding the treatment of cutaneous and articular disease, which was comparable between the biosimilars or following the switch from the reference product to biosimilar therapy. AEs were more prevalent in 1 study following the switch, although there was no control cohort to compare results with.
For patients in the infliximab studies (6 observational and 1 case report), there was comparable effectiveness for the treatment of cutaneous and articular disease between infliximab biosimilars or those switching from infliximab to biosimilar therapy. One study reported an increased number of mild AEs; however, no statistical analysis was conducted.
Limitations of the study included the single-arm designs without controls, the duration of follow-up, and the sample sizes. Additionally, studies did not examine immunogenicity following a switch from the reference product to a biosimilar. Differences in treatment adherences and how patients handle biological drug products are much more likely to cause more changes in outcomes than differences between the innovator product and a biosimilar.
“Understanding that biologics cannot be duplicated (not even from batch to batch of the innovator) makes clear that patients are already switching from one biologic to another, even when they think they are taking the same product,” investigators concluded. “Doing so appears to be safe and effective, and the available real-world evidence suggests that patients can also safely and effectively undergo (non-medical) switching to biosimilar therapies for the treatment of psoriasis.”
Reference:
Ruda RC, Kelly KA, Feldman SR. Real-world outcomes following switching from anti-TNF reference products to biosimilars for the treatment of psoriasis. J Dermatolog Treat. 2023;34(1):2140569. doi:10.1080/09546634.2022.2140569